Articles
Recent Articles
Several key factors determine the curative effect of stem cells
23 Jun at 4:20 pm
Stem cell therapies have emerged as a promising frontier in the realm of regenerative medicine, offering innovative solutions for a diverse range of medical conditions. While the potential of these therapies is vast, their effectiveness is intrinsically tied to a multitude of crucial factors. In this comprehensive exploration, we will delve deep into the fundamental elements that influence the curative effect of mesenchymal stem cell (MSC) treatments. Furthermore, we will dissect the roles played by cell quality, injection route, optimal dosage, and treatment timing in the realm of stem cell therapy.
Understanding Cell Quality
Cell quality plays a pivotal role in determining the success of stem cell therapies. When discussing cell quality, we are referring to the biological potency of a unit or single cell. Essentially, the higher the potency, the better the cell quality. But what exactly is biological potency?
Biological potency, often referred to as bio-efficacy, is a multifaceted notion encompassing two critical facets: strength and effect. While strength relates to the concentration of the treatment, effect pertains to the outcomes achieved at a given strength. To illustrate this concept, let us consider two hypothetical individuals, A and B. A possesses the capability to move 2 tons of bricks daily but can compose only one SCI English sentence within the same timeframe. Conversely, B can transport 1 ton of bricks per day but can produce three SCI English sentences daily. In this scenario, A is more effective at moving bricks, while B exhibits greater efficiency in writing sentences. The concept of efficacy extends beyond stem cell therapies, finding utility in the evaluation of pesticides, drugs, and various biological realms.
In the context of stem cell therapies, biological efficacy assumes a distinct character predicated on the specific indication under consideration. For instance, MSCs manifest both immunosuppressive and angiogenesis-promoting properties, each wielding relevance in the treatment of distinct diseases. Consequently, different biological efficacy indicators are mandated for varying therapeutic applications. However, the current landscape of MSC research remains entrenched in a broader context, necessitating a preliminary focus on the factors underpinning general cell quality.
Several parameters serve as barometers of MSC quality, and these encompass cell viability, donor characteristics, clonogenic ability, cell size, immunosuppressive capacity, and cytokine secretion. In this section, we shall offer a succinct exploration of cell viability and donor characteristics – two pivotal determinants of cell quality:
- Cell Viability: Cell viability constitutes a pivotal facet of cell quality. In essence, cell viability quantifies the percentage of MSCs that retain their vitality before introduction into the patient’s body. Despite its paramount importance, the aspect of cell viability is regrettably often marginalized. Clinical studies divulge a gamut of cell viability rates, oscillating between 70% and upwards of 95%. Astonishingly, a plethora of foreign research articles cite cell viability rates falling short of the 90% threshold. This underscores the critical need to disabuse oneself of the notion that foreign-sourced stem cells inherently exhibit superior quality. The salient importance of cell viability can hardly be overstated, as the survival and functionality of MSCs within the patient’s body are the sine qua non for therapeutic efficacy.
- Donor Characteristics: The characteristics of the donor, particularly age, exert a profound influence on the quality of MSCs. Youthful donors tend to yield MSCs characterized by elevated viability, heightened proliferative potential, and enhanced antioxidant capabilities – attributes that collectively augment their therapeutic potential. Moreover, the source of MSCs, ranging from umbilical cord, cord blood, placenta, deciduous teeth pulp, bone marrow, to fat, constitutes another pivotal variable shaping their quality. Intriguingly, advancing age bestows diminished quantities of MSCs in the bone marrow, making younger sources – such as umbilical cord and cord blood – particularly enticing. It is noteworthy that the gender of the donor can also influence certain functional properties of MSCs, with studies suggesting gender-based differences in immunosuppressive capacity.
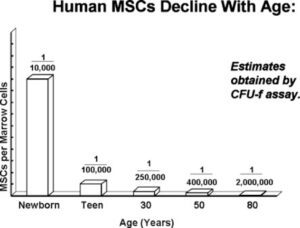
Furthermore, diseases have been demonstrated to impact the functionality of autologous MSCs. For instance, individuals afflicted by autoimmune diseases often harbor bone marrow MSCs afflicted by aberrations in functionality. These include decelerated proliferation, diminished clonogenicity, reduced immunosuppressive capacity, and decreased secretion of growth factors – collectively rendering the patient’s own bone marrow MSCs unsuitable for treating their respective ailments. Theoretically, congenital genetic variations may underlie functional MSC defects, raising intriguing questions regarding the utility of MSCs derived from umbilical cord, umbilical cord blood, and placenta at birth for autologous therapy.
While the foregoing observations underscore the interplay between disease and MSC function, it remains uncertain whether MSC functional defects precede the emergence of disease or vice versa. This captivating conundrum beckons the scrutiny of scientists and researchers alike.
Optimizing Injection Routes
The route of MSC injection represents another pivotal factor profoundly shaping treatment outcomes. Distinct routes of administration engender diverse implications for the distribution and efficacy of MSCs. This segment will scrutinize two principal injection routes: intravenous injection and local interventional injection.
- Intravenous Injection (IV) or Systemic Input
Intravenous injection emerges as the most ubiquitous and straightforward means of administering stem cell therapy. This mode facilitates the infusion of substantial quantities of MSCs with relative ease. However, it harbors a notable drawback – more than 60% of the injected MSCs can fall prey to pulmonary clearance, as a consequence of their dimensions. The unique properties of the pulmonary vasculature permit the passage of particles or cells boasting a diameter smaller than 5 μm while obstructing larger counterparts. Importantly, most MSCs derived from human umbilical cords cluster within the 14-20 μm range – a size class susceptible to pulmonary clearance.
Human umbilical cord blood-derived MSCs exhibit a relatively smoother passage through the pulmonary vasculature compared to their bone marrow counterparts. Strikingly, older donors yield MSCs that more readily domicile in the lungs of mice. Moreover, the number of MSCs retained within the pulmonary confines correlates closely with the expression levels of specific cell surface markers, α4 and α6. Heightened expression levels of these markers correspond to diminished lung retention. However, if MSCs are utilized concomitantly with integrin antibodies, a comprehensive evaluation of the potential risks posed by intravenous integrin antibody administration becomes imperative.
Clinical studies involving the peripheral intravenous infusion of indium-labeled MSCs have yielded intriguing insights. While the initial signals predominantly emanated from the lungs, the signals transitioned predominantly to the spleen and liver after a 48-hour interval. Remarkably, even after ten days of intravenous MSC infusion, at least 50% of the MSCs persisted within the patient’s body, with less than 5% dwelling within the lungs. Such findings spotlight the imperativeness of integrating clinical studies to optimize MSC treatment regimens. From this vantage point, enhancing pulmonary blood oxygen levels becomes a potential avenue to explore, especially within environments boasting standard oxygen content.
- Local Interventional Injection
Local interventional injections encompass a spectrum of approaches, including the integration of biomaterials, intrathecal injections for neurological conditions, and intratracheal injections for respiratory ailments. These techniques are instrumental in enabling MSCs to circumvent the formidable obstacle of pulmonary clearance.
- Intrathecal injection of MSCs represents a prevalent mode of administration for treating neuropathic conditions like stroke, cerebral palsy, and autism. This approach is particularly accommodating for pediatric patients, including premature infants. Studies examining intrathecal umbilical cord-derived MSC injections have reported substantial improvements in motor function among cerebral palsy patients.
Nonetheless, it is imperative to tread cautiously when opting for intrathecal injections under general anesthesia, as they may entail adverse reactions such as fever, vomiting, and, in severe cases, seizures. The origin of these symptoms, in some instances, may be linked to the administration of general anesthesia.
- Intraparenchymal Microinjection: Clinical studies exploring the treatment of cerebral palsy have contemplated the feasibility and efficacy of intrathecal injections combined with cerebral parenchymal microinjections of autologous bone marrow MSCs. While this intervention elicited tangible motor function enhancements in patients, the added benefits of intraparenchymal microinjection were minimal. Notably, adverse events stemming from the intraparenchymal procedure included transient hypothermia and localized pain, with no grave complications arising.
A similar study delving into the local injection of bone marrow MSCs around cerebral ischemic areas among stroke patients reported improved scores in multiple domains. Nevertheless, all patients experienced side effects stemming from the local injection process, including headache, nausea, vomiting, depression, increased muscle tone, fatigue, elevated blood sugar levels, and heightened C-reactive protein levels. This illustrates the need for vigilance when contemplating the microinjection of MSCs into the brain parenchyma.
- Intratracheal Injection: Preterm infants facing bronchopulmonary dysplasia (BPD) represent a group in which MSC intervention has been assessed. Clinical trials among such infants have demonstrated the feasibility and efficacy of MSC intervention. The treatment yielded decreased inflammatory factor concentrations in bronchial secretions, along with marked enhancements in the Respiratory Severity Score.
The delivery of MSCs via endotracheal intubation for mechanical ventilation and surfactant replacement therapy in infants at risk of BPD provides a convenient administration route. Nevertheless, contemporary clinical practice often necessitates the early removal of the endotracheal tube, potentially precluding endotracheal MSC infusion. In such instances, intravenous MSC administration may emerge as a viable alternative.
- Combined with Biological Scaffolds: The integration of stem cells with biological scaffolds represents a novel approach to treating refractory diseases, particularly neurological injuries. Several studies have explored this approach’s potential, revealing promising outcomes. For instance, the combination of autologous bone marrow MSCs with demineralized bone matrix scaffolds achieved substantial bone defect filling within three months.
Intriguingly, the use of donor bone as a growth stimulator may enhance the utility of MSCs for cranial reconstruction. Clinical research conducted at Nanjing Drum Tower Hospital examined the intrauterine transplantation of MSCs combined with collagen scaffolds to treat intrauterine adhesions. The results were encouraging, with numerous patients successfully conceiving and delivering healthy babies.
